Methodology
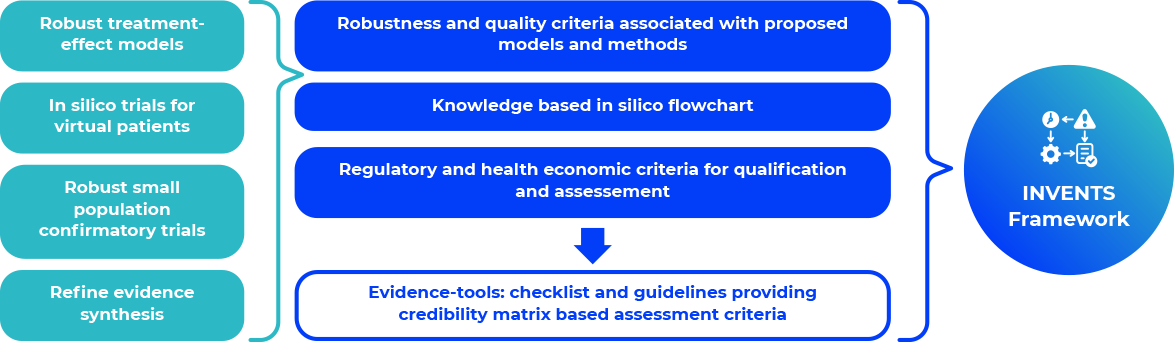
INVENTS aims to generate in silico (trials) based on existing knowledge and computational models under extrapolation approach and geometric distance approach
In 2013, the EU Commission funded three consortia under the call HEALTH.2013.4.2.3 – New methodologies for clinical trials for small population groups. These consortia focused on developing new methodologies for clinical trials in small population groups. While these consortia have published recommended methods, some applied in areas like neonatal seizures and Lyell Syndrome, recent revisions of EU legislation highlight underutilization of scientific and technological advancements. Nearly a decade later, as stated by the EU legislation on medicines for children and rare diseases initiative (EU Law 2021), there’s a recognized need for further development of methods and enhanced support for evaluating evidence from innovative approaches in clinical trials.
Overall methodology
INVENTS methodological development is based on 5 major methodological blocks, all complementary and interconnected. They are supported by 3 blocks including use cases, patients’ engagements and legal perspectives.
INVENTS WPs structure and relation between WPs
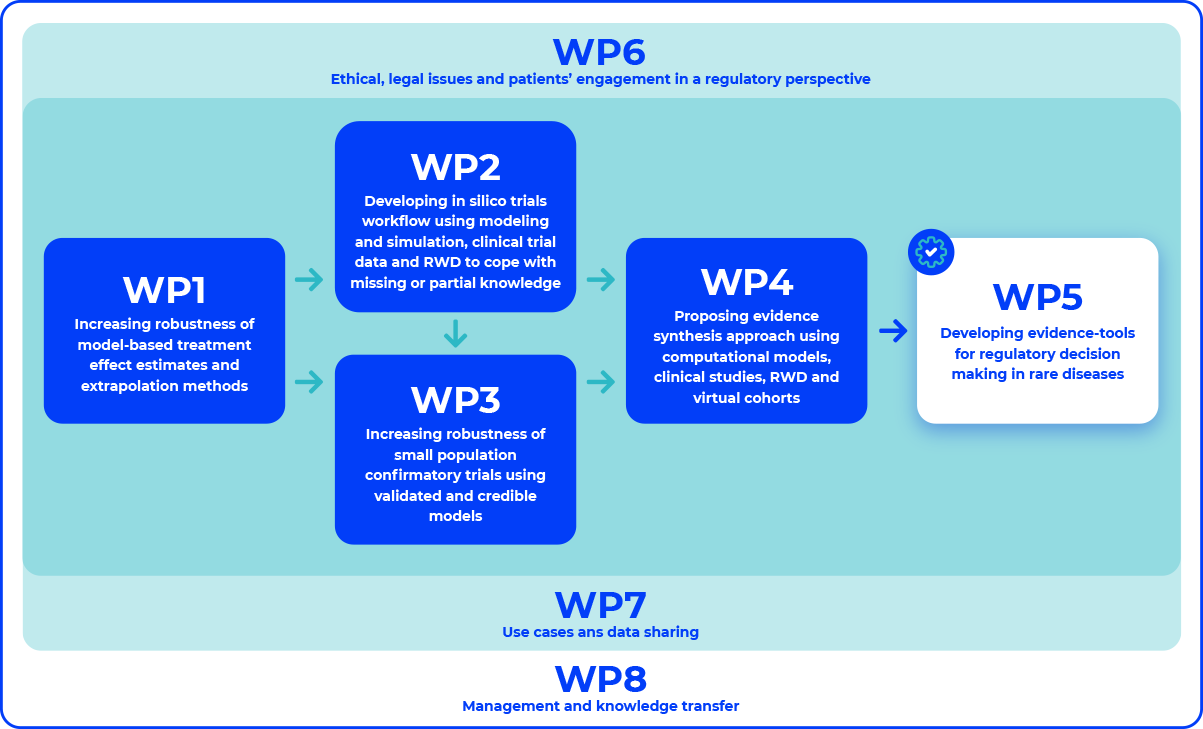
WP1
Increasing robustness of model-based treatment and extrapolation methods
WP2
Cope with missing knowledge
WP3
Increasing robustness of small population confirmatory trials
WP4
Proposing evidence synthesis approach
WP5
Developing evidence tools for regulatory decision making in RD
WP6
Ethical, legal issues and patients’ engagement in a regulatory perspective
WP7
Use cases and data sharing
WP8
Management and knowledge transfer
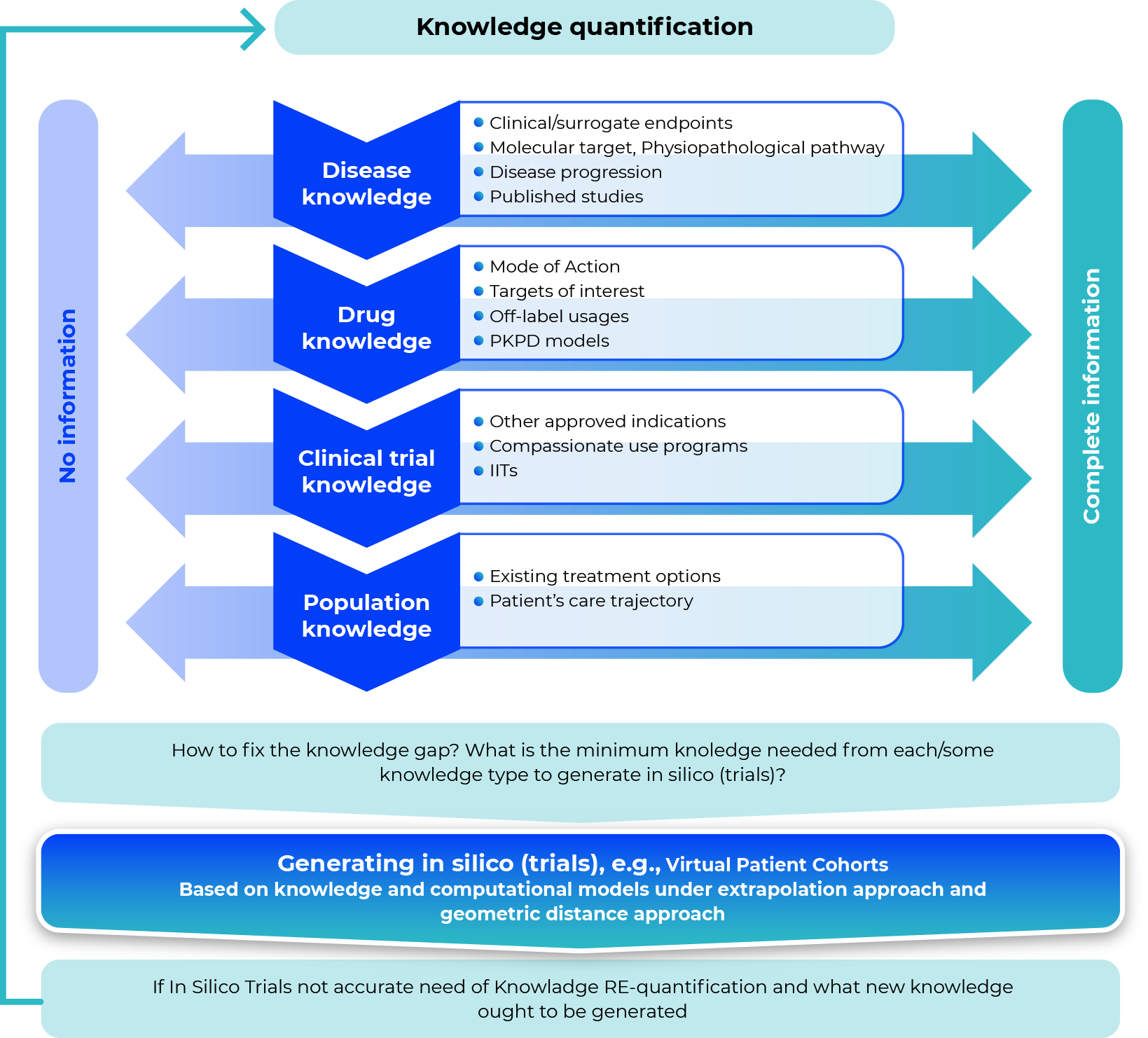
Identifying gaps in the knowledge-based workflow for in silico (trials)
Identifying gaps in the knowledge-based workflow for in silico (trials)
INVENTS framework for decision making