INNOVATIVE DESIGNS, EXTRAPOLATION,
SIMULATION METHODS AND EVIDENCE-TOOLS
FOR RARE DISEASES ADDRESSING REGULATORY NEEDS
The INVENTS program focuses on advancing the future evaluation of treatments and the regulatory decision-making for rare diseases (RD) including paediatric rare diseases.
About rare diseases
European key figures
Up to
36 million
people are concerned in the EU
95%
of rare diseases
have no treatment option
There is more than
6000
distinct rare diseases
70%
of genetic rare diseases
starts in childhood
Challenges
Future assessments for rare diseases treatments face challenges :
A lack of clinical trials and sufficient evidence to decide for treatment
The heterogeneity of rare diseases
Outdated research infrastructures and approaches
In a 5 years project, INVENTS aims to enhance decision-making by incorporating patient experiences, creating in silico evaluation models from clinical trial or real-world data, and developing regulatory decision support tools for regulatory agencies.
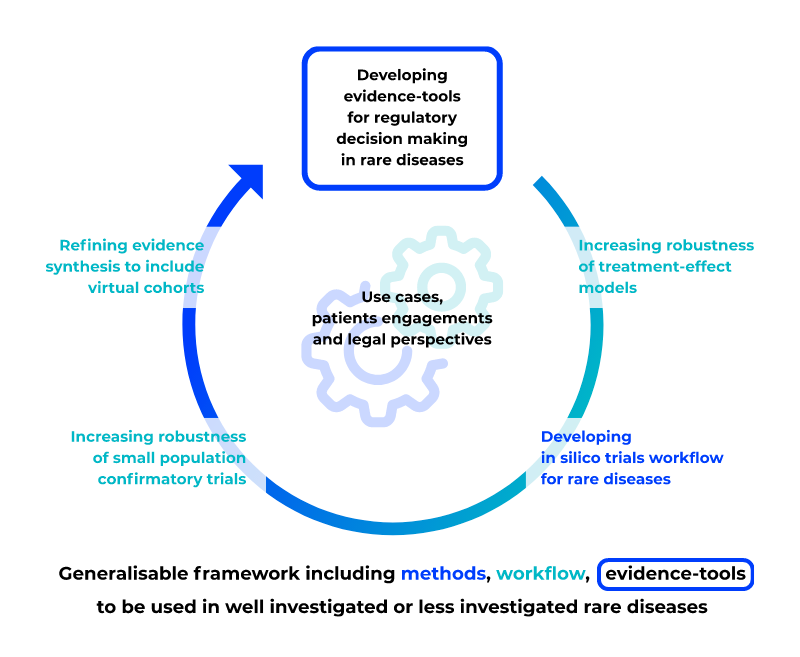
Ambition
INVENTS’ framework will enhance drug development, treatment safety and robustness in rare diseases.


