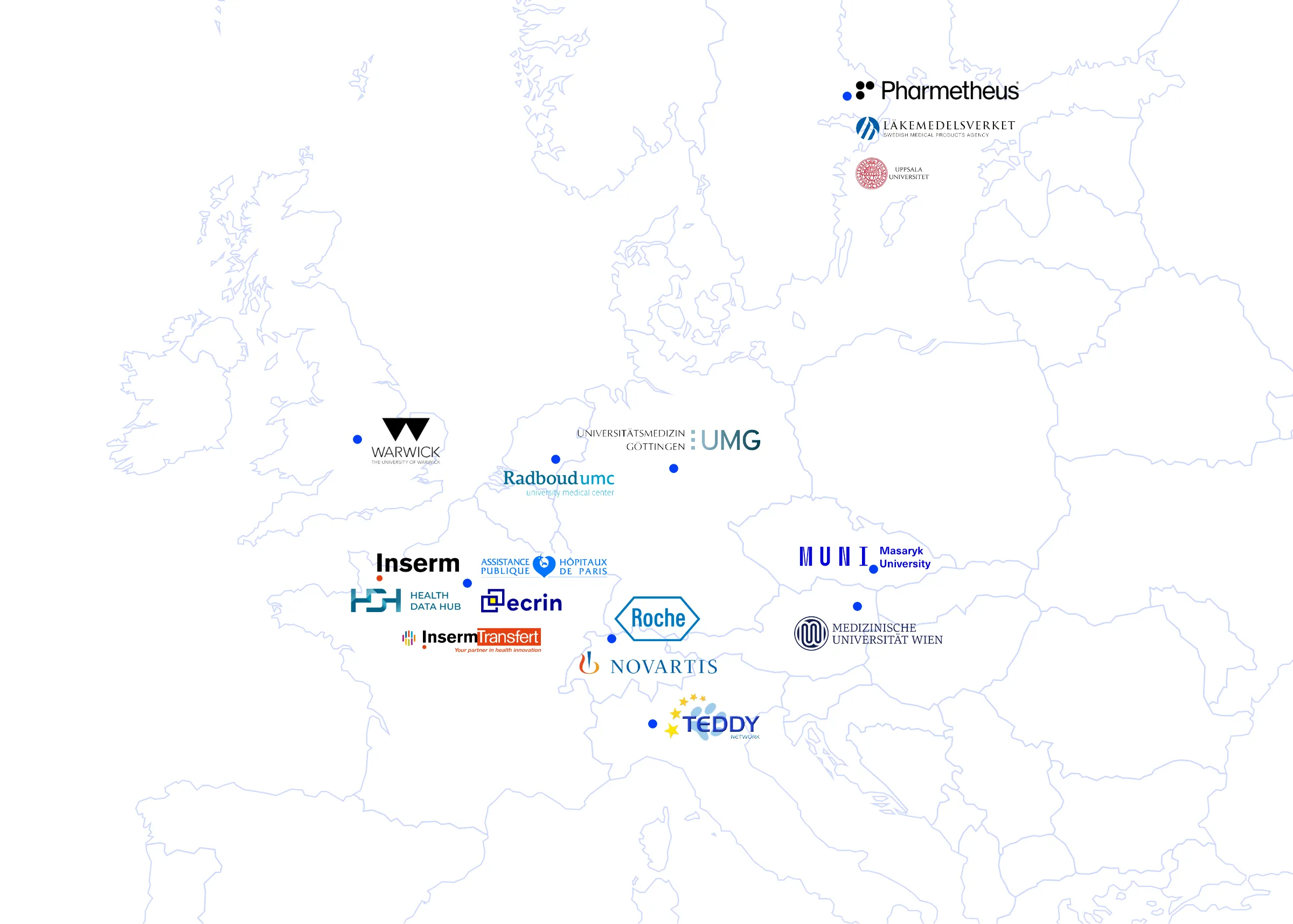
Consortium
- 1 research organization (Inserm)
- 1 platform (French Health Data Hub)
- 2 EU networks (Teddy Network, European Clinical Research Infrastructure Network ECRIN)
- 1 SME (Pharmetheus)
- 2 Pharma companies (Novartis, Roche)
- 1 of the largest RD registries in the world (AP-HP-BNDMR)
- 5 universities/medical university centers (Radboud University Medical Center, Uppsala University, University Medical Center Göttingen, Vienna Medical University, Warwick University)
- 1 technology transfer office (Inserm-Transfert)
- 1 regulatory agency (Swedish Medical Products Agency)
This consortium benefits from an excellent work environment providing cutting-edge platforms for data access provided by Novartis and HDH, as well as access to 27 clinical datasets (provided by Novartis and Roche) and 2 national registries (access provided by APHP-BNDMR).

INSERM – INSTITUT NATIONAL DE LA SANTE ET DE LA RECHERCHE MEDICALE
Paris, France

UU – UPPSALA UNIVERSITET
Uppsala, Sweden

MUW – MEDIZINISCHE UNIVERSITÄT WIEN
Vienna, Austria

HDH – PLATEFORME DES DONNEES DE SANTE
Paris, France

TEDDY – EUROPEAN NETWORK OF EXCELLENCE FOR PEDIATRIC CLINICAL RESEARCH
Pavia, Italy

MUNI – MASARYKOVA UNIVERZITA
Brno, Czechia

MPA – LÄKEMEDELSVERKET
Uppsala, Sweden

ROCHE – F. HOFFMANN-LA ROCHE AG
Basel, Switzerland

RUMC – STICHTING RADBOUD UNIVERSITAIR MEDISCH CENTRUM
Nijmegen, Netherlands

UMG – UNIVERSITY MEDICAL CENTER GÖTTINGEN
Göttingen, Germany

AP-HP – ASSISTANCE PUBLIQUE HÔPITAUX DE PARIS
Paris, France
PMX – PHARMETHEUS AB
Uppsala, Sweden

ECRIN – EUROPEAN CLINICAL RESEARCH INFRASTRUCTURE NETWORK
Paris, France
IT – INSERM TRANSFERT
Paris, France

NOVARTIS – NOVARTIS PHARMA AG
Basel, Switzerland

UOW – THE UNIVERSITY OF WARWICK
Coventry, United Kingdom

INVENTS will propose a comprehensive clinical development framework, starting with enhancing the robustness of model-based treatment effect models through innovative clinical trial designs, as well as evidence synthesis methods augmented and improved by computational models for small sample evidence. These methods and designs will be incorporated into tailored evidence tools for regulatory decision-making.